3. 结构
3.1 二维结构
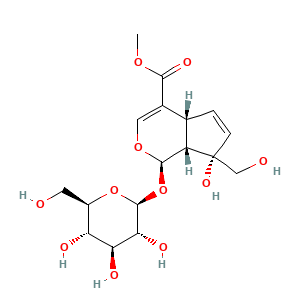
3.2 三维结构
-1
-2
-3
52 54 0 1 0 0 0 0 0999 V2000
-0.5915 -0.8601 0.7367 O 0 0 0 0 0 0 0 0 0 0 0 0
0.3733 -0.0654 -1.2613 O 0 0 0 0 0 0 0 0 0 0 0 0
2.8280 -1.4747 -1.8159 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.1628 0.5770 -0.2172 O 0 0 0 0 0 0 0 0 0 0 0 0
4.1157 -3.8122 -1.0513 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.6642 -2.6896 1.3407 O 0 0 0 0 0 0 0 0 0 0 0 0
-5.2595 -1.3377 1.6830 O 0 0 0 0 0 0 0 0 0 0 0 0
-5.8037 0.3929 -0.5675 O 0 0 0 0 0 0 0 0 0 0 0 0
2.7218 2.7829 1.5595 O 0 0 0 0 0 0 0 0 0 0 0 0
-2.4930 2.4901 -2.1633 O 0 0 0 0 0 0 0 0 0 0 0 0
2.5454 3.4462 -0.6514 O 0 0 0 0 0 0 0 0 0 0 0 0
1.8012 -1.2191 0.4342 C 0 0 1 0 0 0 0 0 0 0 0 0
2.9297 -1.8297 -0.4430 C 0 0 2 0 0 0 0 0 0 0 0 0
2.3537 0.1482 0.9147 C 0 0 1 0 0 0 0 0 0 0 0 0
0.4290 -1.0738 -0.2430 C 0 0 1 0 0 0 0 0 0 0 0 0
4.1487 -1.1601 0.1139 C 0 0 0 0 0 0 0 0 0 0 0 0
3.8354 -0.0872 0.8478 C 0 0 0 0 0 0 0 0 0 0 0 0
1.8950 1.2749 0.0415 C 0 0 0 0 0 0 0 0 0 0 0 0
-1.8803 -0.7768 0.1292 C 0 0 2 0 0 0 0 0 0 0 0 0
2.9916 -3.3495 -0.3254 C 0 0 0 0 0 0 0 0 0 0 0 0
-2.9083 -1.2988 1.1329 C 0 0 1 0 0 0 0 0 0 0 0 0
-4.3352 -1.1054 0.6189 C 0 0 2 0 0 0 0 0 0 0 0 0
-4.5508 0.3192 0.1078 C 0 0 1 0 0 0 0 0 0 0 0 0
-3.4326 0.7260 -0.8546 C 0 0 1 0 0 0 0 0 0 0 0 0
1.0066 1.1086 -0.9455 C 0 0 0 0 0 0 0 0 0 0 0 0
2.4196 2.6271 0.2456 C 0 0 0 0 0 0 0 0 0 0 0 0
-3.5621 2.1831 -1.2870 C 0 0 0 0 0 0 0 0 0 0 0 0
3.2493 4.0636 1.9200 C 0 0 0 0 0 0 0 0 0 0 0 0
1.6774 -1.8445 1.3326 H 0 0 0 0 0 0 0 0 0 0 0 0
2.0364 0.3172 1.9508 H 0 0 0 0 0 0 0 0 0 0 0 0
0.2071 -2.0292 -0.7354 H 0 0 0 0 0 0 0 0 0 0 0 0
5.1646 -1.4696 -0.0886 H 0 0 0 0 0 0 0 0 0 0 0 0
4.5687 0.5561 1.3139 H 0 0 0 0 0 0 0 0 0 0 0 0
-1.9095 -1.4023 -0.7745 H 0 0 0 0 0 0 0 0 0 0 0 0
2.0983 -3.8075 -0.7630 H 0 0 0 0 0 0 0 0 0 0 0 0
3.0893 -3.6769 0.7152 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.7799 -0.8066 2.1042 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.5745 -1.8400 -0.1589 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.5956 1.0125 0.9567 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.4439 0.1024 -1.7589 H 0 0 0 0 0 0 0 0 0 0 0 0
0.6902 1.8851 -1.6292 H 0 0 0 0 0 0 0 0 0 0 0 0
3.5579 -1.9042 -2.2944 H 0 0 0 0 0 0 0 0 0 0 0 0
-4.5068 2.3655 -1.8079 H 0 0 0 0 0 0 0 0 0 0 0 0
-3.4886 2.8538 -0.4244 H 0 0 0 0 0 0 0 0 0 0 0 0
4.1315 -4.7810 -0.9694 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.7720 -3.1455 0.4886 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.0608 -0.7101 2.3988 H 0 0 0 0 0 0 0 0 0 0 0 0
-5.7694 -0.2086 -1.3308 H 0 0 0 0 0 0 0 0 0 0 0 0
-2.5912 3.4226 -2.4206 H 0 0 0 0 0 0 0 0 0 0 0 0
4.1886 4.2486 1.3901 H 0 0 0 0 0 0 0 0 0 0 0 0
2.5200 4.8491 1.7001 H 0 0 0 0 0 0 0 0 0 0 0 0
3.4475 4.0592 2.9951 H 0 0 0 0 0 0 0 0 0 0 0 0
1 15 1 0 0 0 0
1 19 1 0 0 0 0
2 15 1 0 0 0 0
2 25 1 0 0 0 0
3 13 1 0 0 0 0
3 42 1 0 0 0 0
4 19 1 0 0 0 0
4 24 1 0 0 0 0
5 20 1 0 0 0 0
5 45 1 0 0 0 0
6 21 1 0 0 0 0
6 46 1 0 0 0 0
7 22 1 0 0 0 0
7 47 1 0 0 0 0
8 23 1 0 0 0 0
8 48 1 0 0 0 0
9 26 1 0 0 0 0
9 28 1 0 0 0 0
10 27 1 0 0 0 0
10 49 1 0 0 0 0
11 26 2 0 0 0 0
12 13 1 0 0 0 0
12 14 1 0 0 0 0
12 15 1 0 0 0 0
12 29 1 0 0 0 0
13 16 1 0 0 0 0
13 20 1 0 0 0 0
14 17 1 0 0 0 0
14 18 1 0 0 0 0
14 30 1 0 0 0 0
15 31 1 0 0 0 0
16 17 2 0 0 0 0
16 32 1 0 0 0 0
17 33 1 0 0 0 0
18 25 2 0 0 0 0
18 26 1 0 0 0 0
19 21 1 0 0 0 0
19 34 1 0 0 0 0
20 35 1 0 0 0 0
20 36 1 0 0 0 0
21 22 1 0 0 0 0
21 37 1 0 0 0 0
22 23 1 0 0 0 0
22 38 1 0 0 0 0
23 24 1 0 0 0 0
23 39 1 0 0 0 0
24 27 1 0 0 0 0
24 40 1 0 0 0 0
25 41 1 0 0 0 0
27 43 1 0 0 0 0
27 44 1 0 0 0 0
28 50 1 0 0 0 0
28 51 1 0 0 0 0
28 52 1 0 0 0 0
4. 国际命名与标识
4.1 IUPAC Name
methyl (1R,4aR,7S,7aR)-7-hydroxy-7-(hydroxymethyl)-1-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy-4a,7a-dihydro-1H-cyclopenta[c]pyran-4-carboxylate
4.2 InChl
InChI=1S/C17H24O11/c1-25-14(23)8-5-26-15(10-7(8)2-3-17(10,24)6-19)28-16-13(22)12(21)11(20)9(4-18)27-16/h2-3,5,7,9-13,15-16,18-22,24H,4,6H2,1H3/t7-,9+,10-,11+,12-,13+,15+,16-,17+/m0/s1
4.3 InChlKey
XJMPAUZQVRGFRE-ZYEJEXACSA-N
4.4 Canonical SMILES
COC(=O)C1=COC(C2C1C=CC2(CO)O)OC3C(C(C(C(O3)CO)O)O)O
4.5 lsomeric SMILES
COC(=O)C1=CO[C@@H]([C@@H]2[C@H]1C=C[C@]2(CO)O)O[C@H]3[C@@H]([C@H]([C@@H]([C@H](O3)CO)O)O)O
4.6 SDF文件
5. 波谱数据
5.1 13C核磁共振谱(13C NMR)
5.2 1H核磁共振谱(1H NMR)
5.3 质谱(MS)
5.4 红外光谱(IR)
5.5 紫外/可见光谱(UV/Vis)
6. 相关药材
7. 相关靶点
8. 相关疾病